Nanomedicine and Nanoscale Delivery (Focus Group - NND)
(351) An ultra-long-acting prodrug of buprenorphine for opioid dependence
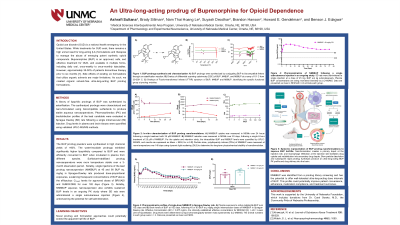
Introduction: Opioid use disorder (OUD) is a national health emergency in the United States. While treatments for OUD exist, there remains an unmet need for long-acting (LA) therapies to manage the abuse of opioids. Buprenorphine (BUP) is an approved, safe, and effective treatment for OUD and is available as daily oral, once-weekly to monthly injectables. However, approximately 35-50% of patients discontinue therapy prior to six months [1]. Side effects of existing LA formulations that utilize organic solvents are major limitations. As such, we created organic solvent-free ultra-LA BUP prodrug formulations.
Learning Objectives:
- Novel prodrug and formulation approaches could potentially extend the apparent half-life of BUP.
Benson Edagwa – Professor, University of Nebraska Medical Center; Howard Gendelman – Professor, University of Nebraska Medical Center; Brady Sillman – Assistant Professor, University of Nebraska Medical Center; Suyash Deodhar – Research Assistant, University of Nebraska Medical Center; Nam Thai Hoang Le – Research Technologist, University of Nebraska Medical Center; Mohammad Ullah Nayan – Research Assistant, University of Nebraska Medical Center; Brandon Hanson – Research Technologist 2, University of Nebraska Medical Center

Ashrafi Sultana, M.Pharm (she/her/hers)
Graduate Research Assistant
University of Nebraska Medical Center
Omaha, Nebraska, United States

