Alternative Methods to Animal Testing
(101) Microstructural Analysis of In Situ Formed Depots for Universal In Vitro Assessment
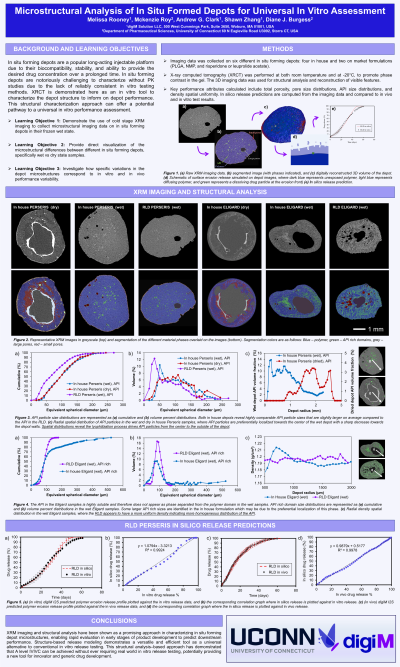
Introduction: In situ forming depots are a popular long-acting injectable platform due to their biocompatibility, stability, and ability to provide the desired drug concentration over a prolonged time1. In situ forming depots are notoriously challenging to characterize without PK studies due to the lack of reliably consistent in vitro testing methods. XRCT is demonstrated here as an in vitro tool to characterize the depot structure to inform on depot performance. This structural characterization approach can offer a potential pathway to a universal in vitro performance assessment.
Learning Objectives:
- Participants will be able to demonstrate XRM imaging to collect microstructural data on iSFD's
- Participants will be able to investigate variations in depot microstructures related to performance
Mckenzie Roy – Graduate Student at the University of Connecticut, Department of Pharmaceutical Sciences, University of Connecticut 69 N Eagleville Road U3092, Storrs CT, USA; Andrew Clark – Director of Pharmaceutical Science, DigiM Solution LLC, 500 West Cummings Park, Suite 3650, Woburn, MA 01801, USA; Shawn Zhang – Co-founder, Managing Director, DigiM Solution LLC, 500 West Cummings Park, Suite 3650, Woburn, MA 01801, USA; Diane Burgess – University of Connecticut Board of Trustees Distinguished Professor of Pharmaceutics, Department of Pharmaceutical Sciences, University of Connecticut 69 N Eagleville Road U3092, Storrs CT, USA

Melissa Rooney
Application Scientist II
DigiM Solution, LLC, United States

