Immuno Delivery (Focus Group - ID)
(245) mRNA formulation development: Looking Beyond the Pandemic
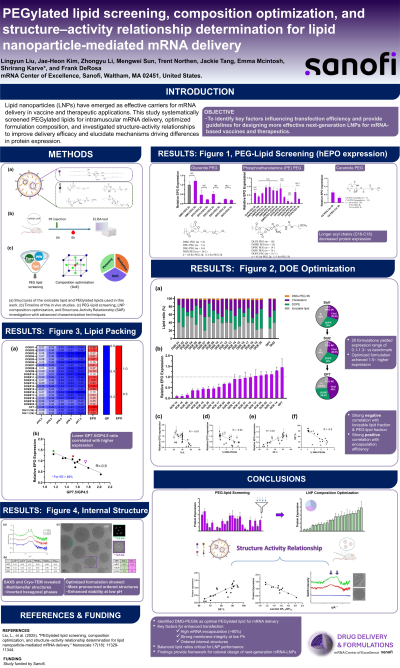
Introduction: The rapid approval of COVID-19 vaccines such as SpikeVax® (Moderna) and Comirnaty® (BioNTech/Pfizer) has thrust mRNA-lipid nanoparticle (LNP) technology into the spotlight (Pardi et al., 2018). With mRNA applications now extending into protein replacement therapy, oncology, gene editing, and treatments for genetic diseases, there is a pressing need to optimize mRNA formulations for broader, long-term use beyond the pandemic.
Learning Objectives:
- List three key mRNA formulation components and describe each component's function.
- Compare PEG lipid variants' effects on mRNA-LNP stability and performance in preclinical data.
- Analyze thermostability data to quantify mRNA product stability improvements.
Shrirang Karve – Global Head of Delivery and Formulations, Formulation and Delivery, Sanofi Pasteur Inc.; Lingyun Liu – Principal Scientist, Formulation and Delivery, Sanofi Pasteur Inc.

Mengwei Sun, PhD
Scientist
Sanofi Pasteur Inc.
Waltham, Massachusetts, United States

