Nanomedicine and Nanoscale Delivery (Focus Group - NND)
(332) Controlled Release of Fluticasone Propionate after Intra-Articular Injection of EP-104IAR in Sheep
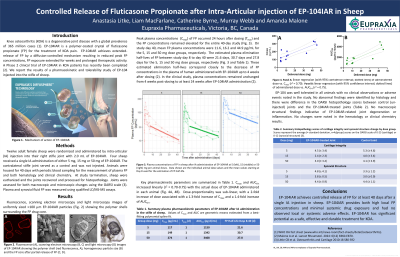
Introduction: Knee osteoarthritis (KOA) is a degenerative joint disease with a global prevalence of 365 million cases [1]. EP-104IAR is a polymer-coated crystal of fluticasone propionate (FP) for the treatment of KOA pain. EP-104IAR achieves extended-release of FP by a diffusion-controlled mechanism resulting in reduced peak FP concentrations, FP exposure extended for weeks and prolonged therapeutic activity. A Phase 2 clinical trial of EP-104IAR in KOA patients has recently been completed [2]. We report the results of a pharmacokinetic and tolerability study of EP-104IAR injected into the stifle of sheep.
Learning Objectives:
- Explain the pharmacokinetic and potential therapeutic properties of extended-release EP-104IAR.
Anastasia Litke, M.Sc. – Senior R&D Program Manager, Eupraxia Pharmaceuticals; Catherine Byrne, Ph.D. – Data Science and Pharmacokinetic Modeling Scientist, Eupraxia Pharmaceuticals; Murray Webb, Ph.D. – VP, Translational Science, Eupraxia Pharmaceuticals; Amanda Malone, Ph.D. – Chief Operations & Scientific Officer, Eupraxia Pharmaceuticals

Liam R. MacFarlane, PhD (he/him/his)
Associate Director of Research and Development
Eupraxia Pharmaceuticals Inc., Canada

